Articles
- Page Path
- HOME > Perspect Integr Med > Volume 3(1); 2024 > Article
-
Protocol
Validation of a New Sham Acupuncture Needle for Double-Blind Trials: A Study Protocol -
Sung Min Lim*

-
Perspectives on Integrative Medicine 2024;3(1):57-60.
DOI: https://doi.org/10.56986/pim.2024.02.008
Published online: February 22, 2024
Department of Clinical Research on Rehabilitation, Korea National Rehabilitation Research Institute, Seoul, Republic of Korea
- *Corresponding author: Sung Min Lim, Department of Clinical Research on Rehabilitation, Korea National Rehabilitation Research Institute, 58 Samgaksan-ro, Gangbuk-gu, Seoul 142-070, Republic of Korea, Email: limsm@outlook.kr
©2024 Jaseng Medical Foundation
This is an open access article under the CC BY-NC license (http://creativecommons.org/licenses/by-nc/4.0/).
- 432 Views
- 10 Download
Abstract
-
Background
- To establish efficacy in acupuncture treatment, rigorous randomized controlled trials (RCT) are needed. Non-invasive sham acupuncture needles are an effective tool for practitioner/participant blinding. This study presents a protocol for the validation of a newly developed sham acupuncture needle.
-
Methods
- A double-blind RCT will be conducted on 66 healthy adults who will be randomly assigned (using computer-generated random numbers) to either the verum (n = 33) or sham (n = 33) acupuncture needle group. The needles will be inserted at 2 acupuncture points: LI4 (upper limb) and ST36 (lower limb). The primary outcome measure is the practitioner/participants belief that they received verum or sham acupuncture. The secondary outcome measures are participant-rated sensations (penetration, pain, and de qi). Adverse events will be recorded with detailed explanations, categorizing occurrences according to related or unrelated to acupuncture. As the newly developed sham acupuncture has not been studied before, an exploratory approach has been adopted. Descriptive statistics, t test, and χ2 test will be applied appropriately.
-
Results
- This study is intended to provide a protocol for the validation of a sham acupuncture needle by using a double-blind RCT setting, and the results will hopefully contribute to the standardization of the needles used for sham acupuncture. The outcomes aim to determine the reliability of practitioner/participant blinding, participant experience of sensations, and lay groundwork for a standardized control group for clinical trials in the future. The newly developed non-invasive sham acupuncture needle may reduce bias and improve reliability in the size effect of acupuncture treatment.
-
Trial Registration
- Clinical Research Information Service of the Republic of Korea (registration no.: KCT0008335, https://cris.nih.go.kr).
- Acupuncture is an invasive treatment. To determine the efficacy of acupuncture treatment non-invasive sham acupuncture needles are needed to enable blinding for randomized controlled trials (RCTs) [1,2]. Currently, the Streitberger needle [3] and Park-sham acupuncture needle [4] are used in clinical trials. To ensure practitioner/participant blinding in clinical trials, sham acupuncture should not be visibly distinguishable from acupuncture treatment, and it should feel similar to the participant, yet lack the same physiological effects [5,6]. Non-penetrating sham acupuncture needles have a blunt (rather than a sharp) end to prevent the needle from penetrating the skin, ensuring blinding of the practitioner/participant, who cannot distinguish between verum and sham acupuncture needles [7–9].
- The development of a valid sham acupuncture model is crucial in establishing a gold standard placebo for future acupuncture studies. Furthermore, for acupuncture treatment to meet high scientific standards, the double-blind RCTs of acupuncture must demonstrate safety, low bias risk, and, ultimately efficacy, where acupuncture treatment must be superior to the sham acupuncture control.
- Therefore, this study presents a protocol for a verification study, to validate a new sham acupuncture needle for double-blinded RCTs, for a rigorous evaluation of acupuncture effectiveness.
Introduction
- 1. Study design
- A double-blind RCT will be conducted to determine whether the newly developed sham acupuncture needle facilitates practitioner/participant blinding compared with an acupuncture needle. The study protocol, including the informed consent form, has been approved by the Institutional Review Board of the Korean National Rehabilitation Center (approval no.: NRC-2023-01-010) and has been registered with the Clinical Research Information Service of the Republic of Korea (identification no.: KCT0008335).
- 2. Participants
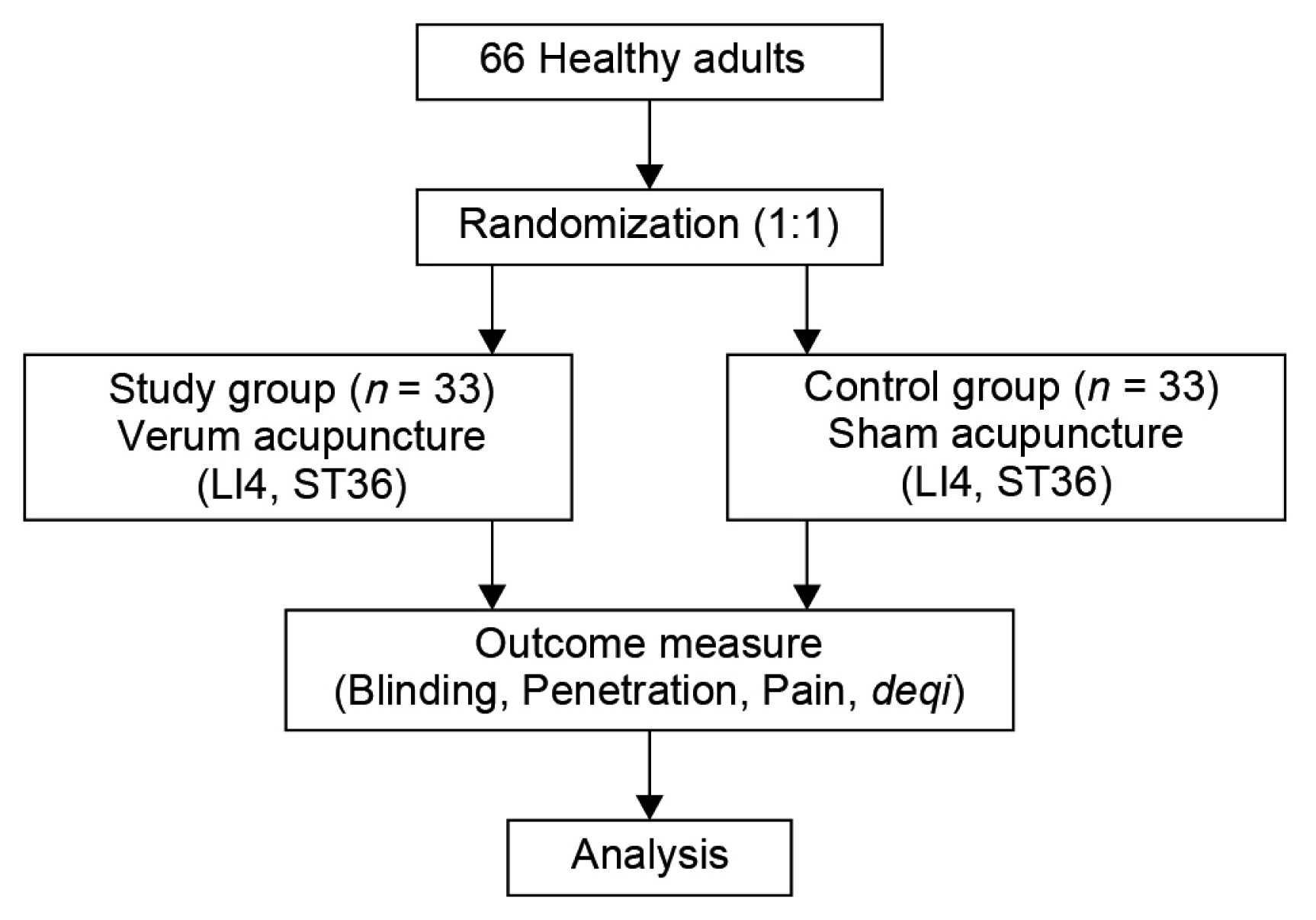
- A total of 66 healthy adults will be enrolled in the study and randomly assigned to the verum acupuncture needle group (n = 33) or sham acupuncture needle group (n = 33; Fig. 1). The inclusion criteria are: aged ≥ 19 years, and provision of informed consent to participate in the study. The exclusion criteria are: women who are pregnant or breastfeeding, and other individuals deemed inappropriate for the trial by the principal or sub-investigator.
- 3. Randomization and allocation
- The participants will be allocated to the sham or verum acupuncture needle group in a 1:1 ratio. Randomization will be performed using computer-generated random numbers, and the allocation results will be placed in an opaque sealed envelope.
- 4. Blinding
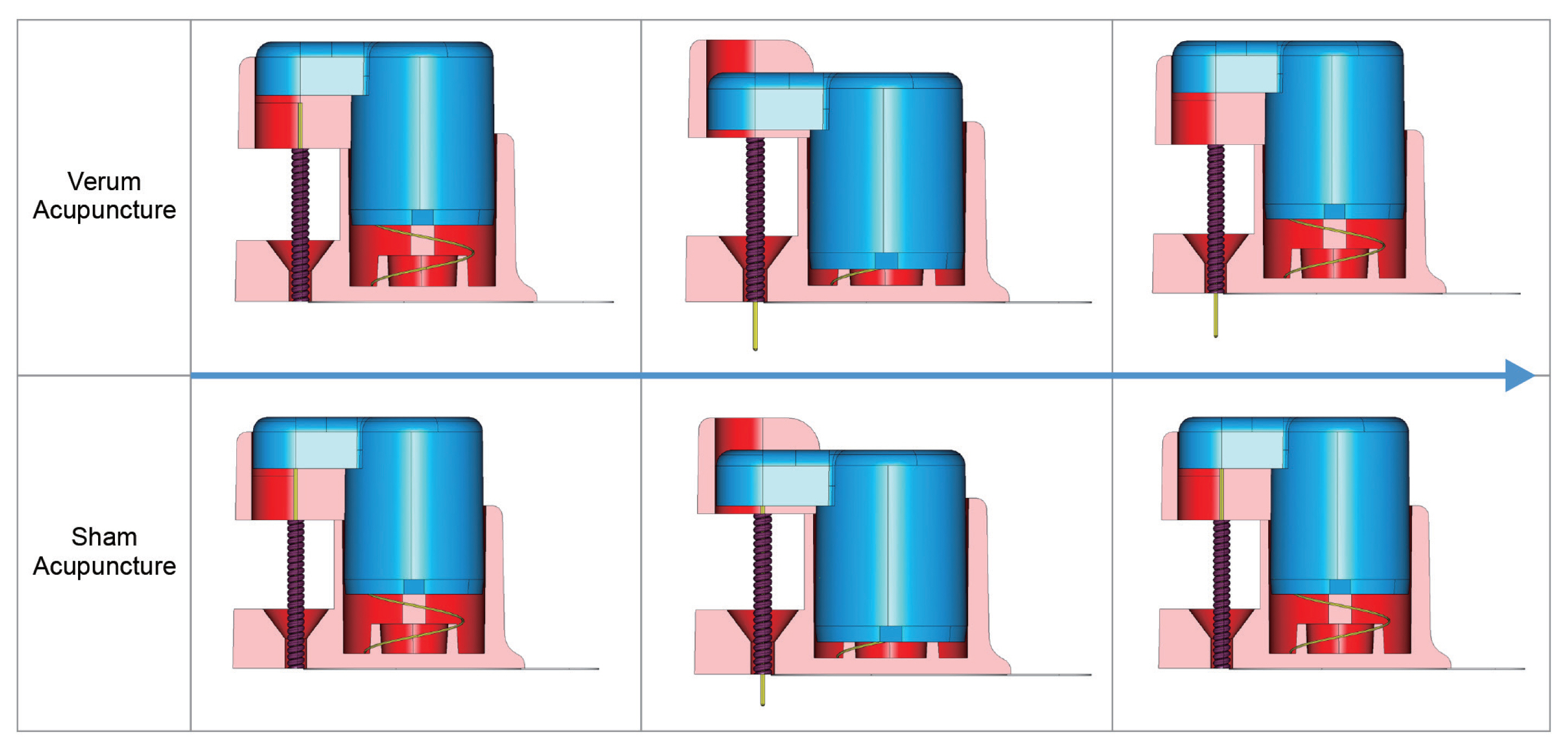
- Participants will be blinded to the acupuncture treatment they receive and the practitioner will be blinded to the acupuncture they deliver. The verum or sham acupuncture needles look identical and the delivery system is identical (Fig. 2).
- 5. Interventions
- The participants will be asked to lie down on the treatment bed for approximately 5 minutes before receiving the verum or sham acupuncture. The needles will be inserted at 2 acupuncture points: LI4 (upper limb) and ST36 (lower limb). These points have been selected because they are the most commonly used acupuncture points in previous studies on the validation of sham acupuncture.
- 6. Outcome measures
- After the procedure has been performed, whilst maintaining blinding, the participants will be asked whether they thought the treatment that they had received was using verum acupuncture needles or sham acupuncture needles. Moreover, the practitioner will be asked whether they thought the treatment they have administered was performed using verum acupuncture needles or sham acupuncture needles.
- The participants will be asked to indicate the sensation of needle penetration, pain, and de qi following their verum or sham acupuncture, using an 11-point scale from 0 (no sensation/pain at all) to 10 (cannot bear the sensation/pain)
- 7. Safety assessment
- All adverse events (AEs) that may occur during the study will be recorded with detailed explanations including the frequency of AEs and whether the AEs were related to sham or verum acupuncture.
- 8. Sample size calculation
- Since the newly developed sham acupuncture needle has not yet been researched, it is difficult to predict the magnitude of the effect. Considering the work by Takakura et al [10] who had performed a double-blind sham acupuncture validation study with 60 healthy adults divided into [a verum acupuncture group (n = 30) and a sham acupuncture group (n = 30)]. It was decided that the target sample size for this study would be 66 participants [verum acupuncture group (n = 33) and sham acupuncture group (n = 33)] accounting for dropout rate.
- 9. Statistical analysis
- Statistical analysis will be performed using the SPSS Version 21.0 (IBM Corp., Armonk, NY, USA) software, and the significance level will be set at 5%. Continuous data will be presented using descriptive statistics (mean and standard deviation), and categorical data will be presented as frequency and percentage. For continuous data, intergroup comparisons will be performed using the independent t test, and for categorical variables, the χ2 test will be used.
- For the primary outcome, the Bang blinding index [11] will be used to determine whether a response infers blinding and the 95% confidence interval will be used. The Bang Blinding Index assesses the success of blinding in RCTs to calculate blinding per treatment site. Participants will be assigned to random, opposite, and unblinded groups, to describe their blinding status. For the secondary outcome analysis, the sensation of the penetration, pain, and de qi sensation will be compared between groups using an independent t test. For the safety evaluation, all AEs that may occur, along with a detailed description and the frequency of AEs either related or unrelated to the sham or verum acupuncture needle group will be recorded. The number of cases of AEs related to the verum acupuncture needle will be compared with the sham acupuncture needle group using the χ2 test.
Materials and Methods
6.1. Primary outcome measure
6.2. Secondary outcome measures
- To determine the effectiveness of acupuncture treatment, RCTs that robustly control for the placebo effect are needed. The difficulty lies in the delivery of physiologically inert sham acupuncture treatment which is indistinguishable from acupuncture treatment [12]. Currently, the Streitberger and Park-sham acupuncture needles are being used as sham (or control) needles for this purpose in blind trials [13]. To improve the reliability in the size effect and provide a more rigorous RCT setting, double-blind trials are necessary.
- In this protocol a double-blind RCT will be performed which is unlike previous studies using sham acupuncture. The sham acupuncture needles will allow the practitioner to apply similar physical and visual stimulus during the procedure, facilitating the blinding of both practitioner and participant.
- The Takakura needle has been previously reported in 2007 to enable double-blinded acupuncture [10]. However, it has not been commercialized, leaving the field awaiting the standardization of double-blind acupuncture trial tools [13].
- This validation study provides a protocol for the validation of a newly developed double-blind acupuncture trial where sham acupuncture needles are used. The study will be conducted on healthy adults, where treatment will be blinded for the participant and practitioner whereby the participant sensation of pain, penetration, and de qi will be measured. A comparison of verum acupuncture and sham acupuncture approaches will be compared. The outcome of this proposed validation study may contribute to the standardization of sham acupuncture used in acupuncture research.
Discussion
-
Conflicts of Interest
The author has no conflicts of interest to declare.
-
Funding
This work is supported by a grant from the National Research Foundation of Korea funded by the Korean government (Ministry of Science and ICT; 2019R1F1A1058183, 2021R1F1A1045514). The funders have no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
-
Ethical Statement
This study has been approved by the Institutional Review Board of the National Rehabilitation Center (no.: NRC-2023-01-010). Informed consent for participation in the study will be obtained from all participants.
Article information
Data Availability
- [1] World Health Organization [Internet]. WHO benchmarks for the practice of acupuncture: World Health Organization: 2020 Available from: https://apps.who.int/iris/handle/10665/340838
- [2] White AR, Filshie J, Cummings TM. International Acupuncture Research Forum. Clinical trials of acupuncture: consensus recommendations for optimal treatment, sham controls and blinding. Complement Ther Med 2001;9(4):237−45.PubMed
- [3] Streitberger K, Kleinhenz J. Introducing a placebo needle into acupuncture research. Lancet 1998;352(9125):364−5.ArticlePubMed
- [4] Park J, White A, Stevinson C, Ernst E, James M. Validating a new non-penetrating sham acupuncture device: two randomized controlled trials. Acupunct Med 2002;20(4):168−74.ArticlePubMedPDF
- [5] Lund I, Näslund J, Lundeberg T. Minimal acupuncture is not a valid placebo control in randomised controlled trials of acupuncture: a physiologist’s perspective. Chin Med 2009;4:1. ArticlePubMedPMCPDF
- [6] Zhang CS, Tan HY, Zhang GS, Zhang AL, Xue CC, Xie YM. Placebo devices as effective control methods in acupuncture clinical trials: A systematic review. PLoS One 2015;10(11):e0140825.. ArticlePubMedPMC
- [7] Zhu D, Gao Y, Chang J, Kong J. Placebo acupuncture devices: considerations for acupuncture research. Evid Based Complement Alternat Med 2013;2013:628907. ArticlePubMedPMCPDF
- [8] Linde K, Niemann K, Schneider A, Meissner K. How large are the nonspecific effects of acupuncture? A meta-analysis of randomized controlled trials. BMC Med 2010;8:75. ArticlePubMedPMCPDF
- [9] Paterson C, Dieppe P. Characteristic and incidental (placebo) effects in complex interventions such as acupuncture. BMJ 2005;330(7501):1202−5.ArticlePubMedPMC
- [10] Takakura N, Yajima H. A double-blind placebo needle for acupuncture research. BMC Complement Altern Med 2007;7:31. ArticlePubMedPMCPDF
- [11] Bang H, Ni L, Davis CE. Assessment of blinding in clinical trials. Control Clin Trials 2004;25(2):143−56.ArticlePubMed
- [12] Moroz A, Freed B, Tiedemann L, Bang H, Howell M, Park JJ. Blinding measured: a systematic review of randomized controlled trials of acupuncture. Evid Based Complement Alternat Med 2013;2013:708251. ArticlePubMedPMCPDF
- [13] Lim SM. Trends in the development of sham acupuncture-related technologies focused on patents applied for in South Korea. Perspect Integr Med 2023;2(1):36−41.ArticlePDF




 PubReader
PubReader ePub Link
ePub Link Cite
Cite