Search
- Page Path
- HOME > Search
Original Article
- Acupuncture Needles and the Risk of Lymphedema After Breast Cancer Surgery: A Retrospective National Cohort Study
- Ye-Seul Lee, Yucheol Lim, Jiyoon Yeo
- Perspect Integr Med. 2024;3(1):29-36. Published online February 22, 2024
- DOI: https://doi.org/10.56986/pim.2024.02.004
- 1,864 View
- 28 Download
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material 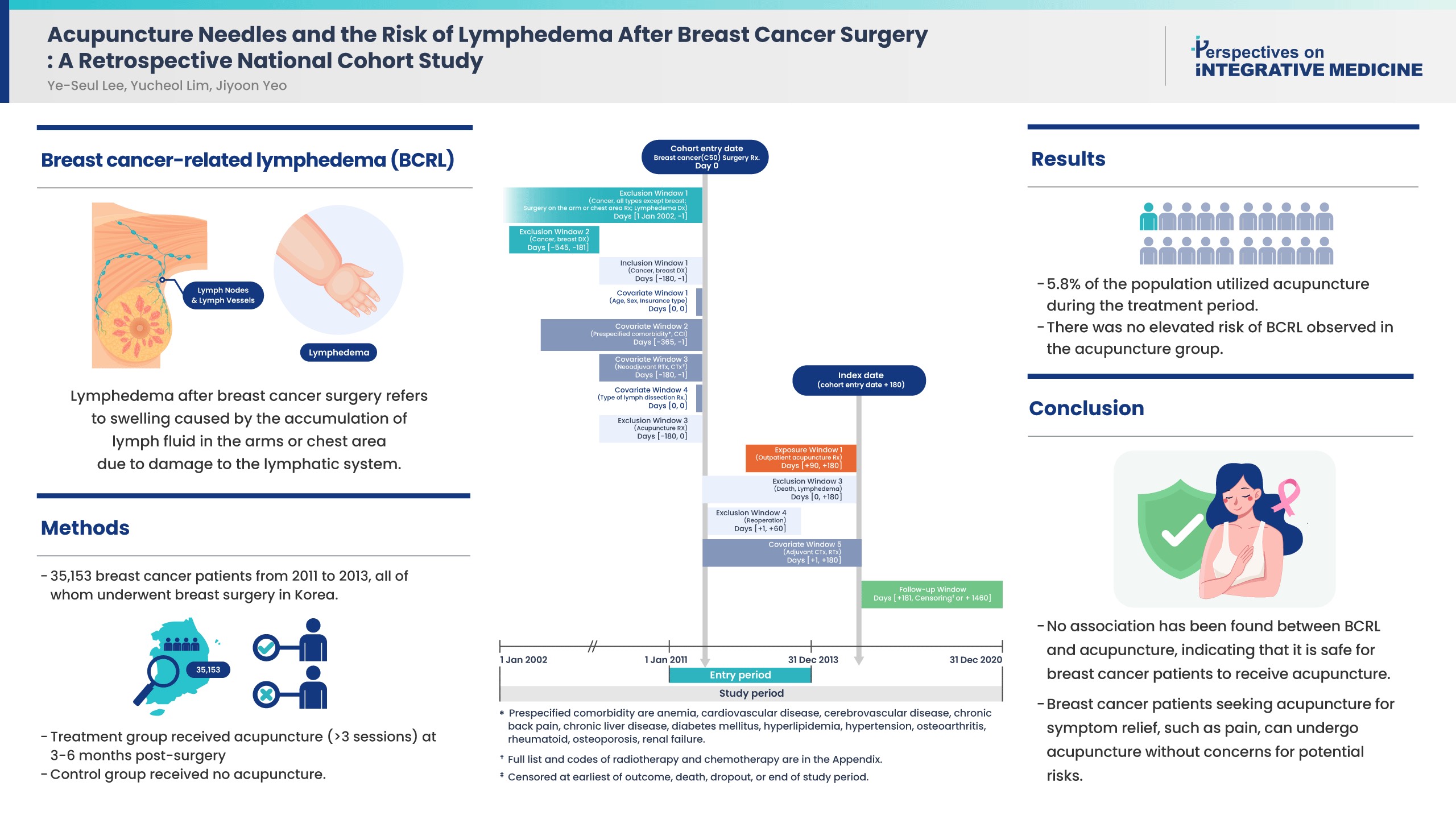
- Background
Controversies remain over the impact of using needles on breast cancer patients after surgery due to risk of breast cancer-related lymphedema (BCRL). While recent literature suggests that vascular access during the postsurgical stage does not affect the risk of BCRL, the impact of acupuncture on the risk of BCRL during the postsurgical stage has not been studied.
Methods
This study included 35,153 patients from 2011 to 2013 who were newly diagnosed with breast cancer in a population-based cohort from the Korean National Health Insurance Service database. All patients received breast surgery, and the treatment group received acupuncture for more than 3 sessions in the 3-6 months post-surgery. The control group did not receive acupuncture. The incidence rate ratio, Kaplan-Meier curve, and Cox proportional hazards models were used to compare the risk of BCRL, and death between groups.
Results
About 5.8% of the study population received acupuncture during the 3-6 months post-surgery treatment window. After propensity score matching, the acupuncture treatment group did not show an increased risk of BCRL (IRR 1.017, 95% CI 0.868-1.193; unadjusted HR 1.018, 95% CI 0.868-1.193). This risk was robust in all multivariate Cox proportional hazards models.
Conclusion
An association of BCRL with acupuncture was not observed. Patients who received acupuncture to manage symptoms such as pain during the 3-6 months postsurgical stage did not have a higher risk of developing BCRL. Breast cancer patients who seek acupuncture to alleviate post-surgery symptoms such as pain, can receive acupuncture without concerns for potential risk of BCRL.
Guideline
- ACURATE: A Guide for Reporting Sham Controls in Trials Using Acupuncture
- Ye-Seul Lee, Song-Yi Kim, Hyangsook Lee, Younbyoung Chae, Myeong Soo Lee
- Perspect Integr Med. 2023;2(2):100-106. Published online June 23, 2023
- DOI: https://doi.org/10.56986/pim.2023.06.004
- 1,329 View
- 20 Download
-
 Abstract
Abstract
 PDF
PDF - This paper presents the Acupuncture Controls gUideline for Reporting humAn Trials and Experiments (ACURATE) checklist, an extension of The Consolidated Standards for Reporting of Trials (CONSORT) and to be used along with STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA) when both real and sham acupuncture needles are used in the study. This checklist focuses on a clear depiction of sham needling procedures to enhance replicability and enable a precise appraisal. We encourage researchers to use ACURATE in trials and reviews involving sham acupuncture to assist reporting of sham acupuncture procedures and the related components.
Review Article
- Minimum Clinically Important Difference for Nonsurgical Interventions for Spinal Diseases: Choosing the Appropriate Values for an Integrative Medical Approach
- Ye-Seul Lee, Sungmin Lee, Yoon Jae Lee, In-Hyuk Ha
- Perspect Integr Med. 2023;2(2):86-99. Published online June 23, 2023
- DOI: https://doi.org/10.56986/pim.2023.06.003
- 910 View
- 26 Download
- 2 Citations
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material 
- The minimal clinically important difference (MCID) plays a crucial role in the design and interpretation of clinical trials, as it helps in distinguishing between statistically significant and clinically meaningful outcomes. This scoping review aims to collate and appraise the current research concerning the validation of MCIDs for surgical and nonsurgical measures for spine disorders. Two databases of MEDLINE (PubMed and EMBASE) were searched. There were 1,590 studies retrieved and 79 were selected as eligible for review. Measurement tools such as the Oswestry Disability Index, Neck Disability Index, Numeric Rating Scale, and Visual Analogue Scale were assessed by regions and interventions. A total of 24 studies identified MCIDs on nonsurgical interventions, and 55 studies identified MCIDs on surgical interventions. The range of MCIDs varied greatly depending on study population, specific interventions, calculation methods, and outcomes. This scoping review emphasizes the complexity and variability in determining MCIDs for musculoskeletal or neurodegenerative spinal diseases, influenced by several factors including the intervention type, measurement tool, patient characteristics, and disease severity. Given the wide range of reported MCIDs, it is crucial to consider the specific context when interpreting these values in clinical and research settings. To select an appropriate MCID value for comparison in a clinical trial, careful consideration of the patient group, intervention, assessment tools, and primary outcomes is necessary to ensure that the chosen MCID aligns with the research question at hand.
-
Citations
Citations to this article as recorded by- Minimum clinically important difference and substantial clinical benefit in patients with chronic temporomandibular disorders
Jaemin Son, Eun‐San Kim, Yoon Jae Lee, Nam‐Woo Lee, In‐Hyuk Ha
Journal of Oral Rehabilitation.2024;[Epub] CrossRef - Safety and effectiveness of integrative Korean medicine for the management of patients sustaining injuries in traffic accidents during pregnancy: A retrospective chart review and questionnaire survey
Dahyun Kyung, Kyoung Sun Park, Ji-Eun Koo, Sujin Kim, Jiwon Park, Jun-Hyo Bae, Jieun Bae, Suna Kim, Yoon Jae Lee, In-Hyuk Ha
Medicine.2024; 103(21): e38250. CrossRef
- Minimum clinically important difference and substantial clinical benefit in patients with chronic temporomandibular disorders



 First
First Prev
Prev


