Search
- Page Path
- HOME > Search
Review Articles
- Effectiveness and Safety of Low-Level Laser Treatment for Lumbar Disc Herniation: A Systematic Review and Meta-Analysis
- Sang Jun Lee, Seung Jin Noh, Jeong Rock Kim, Kyung Bok Park, Sae-rom Jeon, Yejin Hong, Dongwoo Nam
- Perspect Integr Med. 2023;2(3):155-163. Published online October 23, 2023
- DOI: https://doi.org/10.56986/pim.2023.10.003
- 1,536 View
- 46 Download
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material 
- Background
Low-level laser treatment (LLLT) is used to treat low back pain (LBP) however, its effects on lumbar disc herniation (LDH) remain unclear. The safety and effectiveness of LLLT for LDH was determined using a systematic review of randomized clinical trials.
Methods
Studies on LLLT in adults with LDH were identified from 12 worldwide databases. A risk of bias assessment and a meta-analysis with categorization according to the type of control used (inactive, active, or add-on) was performed. The quality of evidence was assessed using the Grading of Recommendations, Assessment, Development, and Evaluation.
Results
The quantitative analyses included five studies. LLLT was significantly more effective at treating LDH [leg pain visual analog scale (VAS) mean difference (MD): -1.90, 95% confidence interval (CI): -2.01, -1.80, I2 80%; LBP VAS MD: -0.79, 95% CI: -0.87, -0.71, I2 80%] than inactive controls (placebo or sham). The quality of the evidence ranged from “low” to “very low.” As an add-on to usual care, LLLT significantly improved pain intensity and disability compared with usual care (leg pain VAS MD: -2.52, 95% CI: -2.65, -2.40, I2 97%; LBP VAS MD: -1.47, 95% CI: -1.58, -1.36; Oswestry Disability Index MD: -4.10, 95% CI: -4.55, -3.65, I2 6%). However, the quality of the evidence ranged from “moderate” to “low.”
Conclusion
LLLT significantly improved outcomes compared with the inactive controls, but was not more effective than usual care for LDH. In combination with usual care, LLLT was significantly more effective than usual care alone highlighting the potential of LLLT.
- An Umbrella Review of Systematic Reviews for Chuna (or Tuina) Manual Therapy on Musculoskeletal Disorders
- Doori Kim, Gil Geun Baek, Byung-Cheul Shin
- Perspect Integr Med. 2023;2(3):142-154. Published online October 23, 2023
- DOI: https://doi.org/10.56986/pim.2023.10.002
- 766 View
- 30 Download
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material 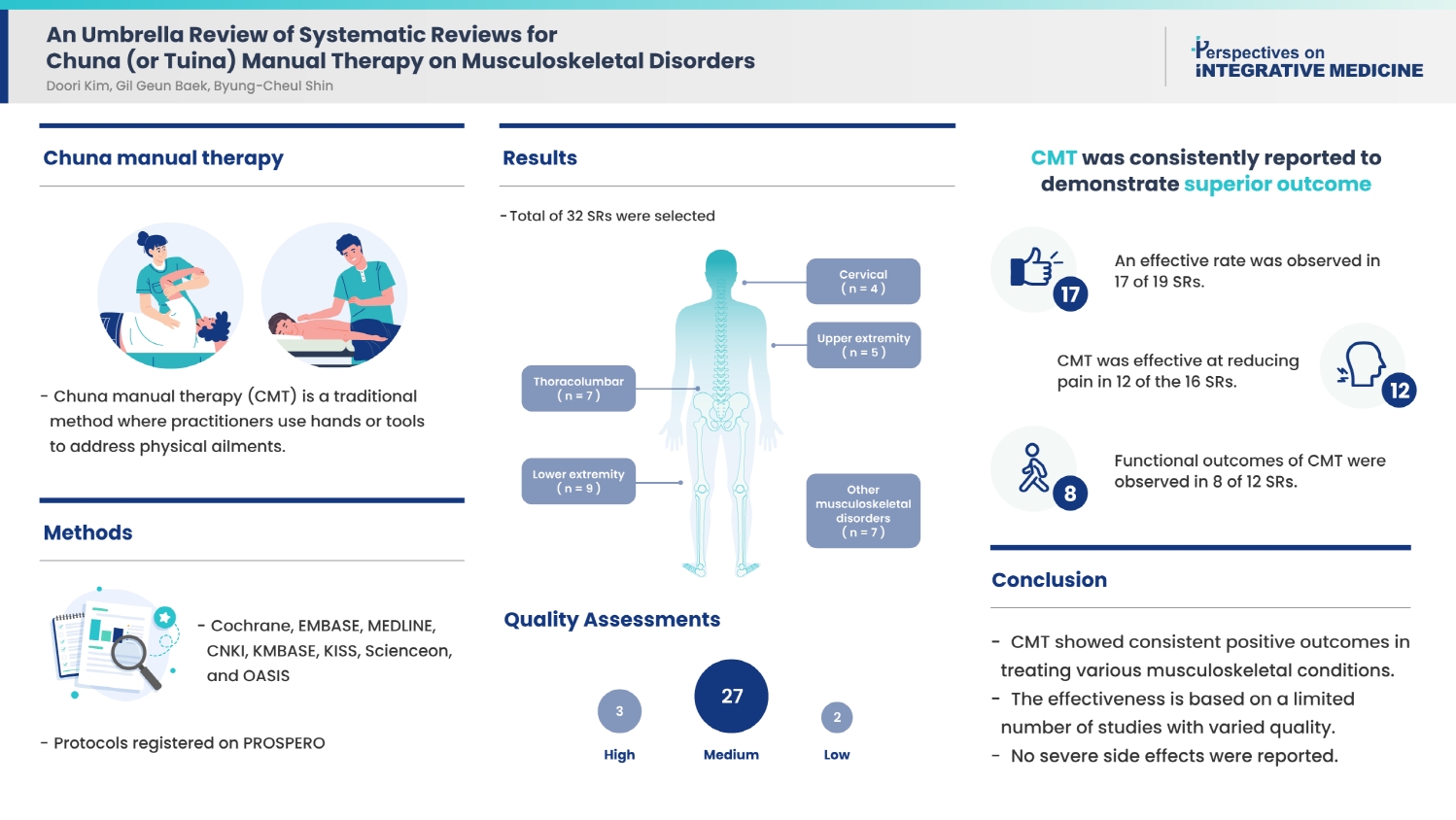
- Background
To provide clinicians with reliable evidence an umbrella review of systematic reviews (SRs) on Chuna manual therapy (CMT) for musculoskeletal disorders was performed to synthesize important outcomes.
Methods
There were eight databases (Cochrane, EMBASE, MEDLINE, CNKI, KMBASE, KISS, Scienceon, and OASIS) searched as well as the international database Prospective Register of Systematic Reviews in health and social care until August 2023. SRs of randomized controlled trials involving patients with musculoskeletal conditions, limited to interventions explicitly labeled as “Chuna” or “Tuina” in English, Chinese, or Korean language were retrieved. Two reviewers independently conducted selection and data extraction, and SR quality was assessed using A Measurement Tool to Assess Systematic Reviews tool (low, medium, or high quality).
Results
This review included 32 SRs, categorized by cervical (n = 4), thoracolumbar (n = 7), upper extremity (n = 5), lower extremity (n = 9), and other musculoskeletal disorders (n = 7). Quality assessments determined that three SRs were of “high” quality, two were “low” quality, and the remaining SRs were of “medium” quality. CMT was consistently reported to demonstrate superior outcomes: an effective rate was observed in 17 of 19 SRs, CMT was effective at reducing pain in 12 of the 16 SRs, and functional outcomes of CMT were observed in 8 of 12 SRs. No serious adverse events were reported.
Conclusion
CMT may be a safe and effective treatment for various musculoskeletal disorders based on the limited number of studies and the low quality of included SRs.
Protocols
- Effectiveness and Safety of Duantengyimu-tang for Rheumatoid Arthritis: A Protocol for a Systematic Review and Meta-Analysis
- Gyoungeun Park, Jeong-Hyun Moon, Eun-Jung Kim, Won-Suk Sung
- Perspect Integr Med. 2023;2(2):134-137. Published online June 23, 2023
- DOI: https://doi.org/10.56986/pim.2023.06.009
- 732 View
- 11 Download
-
 Abstract
Abstract
 PDF
PDF - Background
Per-oral pharmacological medication is a representative treatment for rheumatoid arthritis (RA), and has improved over several guidelines. However, limitations of long-term use of these medications including adverse events, led to the introduction and utilization of complementary and alternative treatments for RA. Several herbal medicine decoctions have been reported to be effective and safe; a recent study introduced Duantengyimu-tang (DTYMT). Regardless of the pharmacological effects of the DTYMT components, there are concerns about its safety. Therefore, this systematic review (SR) will focus on the effectiveness and safety of DTYMT treatment for RA.
Methods
Searches for randomized controlled trials using DTYMT treatment for RA will be performed using multiple electronic databases, manual searches, and emails (if necessary). A summary will be written using data on outcome measurements of the study participants, interventions, adverse events, and risk of bias in the studies. The primary outcomes will be disease activity scores including effective rate, tender joints, swollen joints, and morning stiffness. The secondary outcomes will include adverse events and blood tests for RA (erythrocyte sedimentation rate, C-reactive protein, and rheumatoid factors). This SR will use Review Manager software to perform a meta-analysis, the Cochrane Collaboration “risk of bias” tool, and determine the quality of evidence using the Grades of Recommendation, Assessment, Development, and Evaluation method.
Results
This SR will investigate the clinical effectiveness and safety of DTYMT treatment in patients with RA.
Conclusion
This SR aims to be informative for patients and clinicians in clinical practice, researchers, and policymakers in managing RA.
- Systematic Review Protocol for Sham Acupuncture Validation Research
- Sung Min Lim
- Perspect Integr Med. 2023;2(2):131-133. Published online June 23, 2023
- DOI: https://doi.org/10.56986/pim.2023.06.008
- 684 View
- 8 Download
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 
- Background
Streitberger and Park sham needles have been developed and used as non-penetrating sham acupuncture needles that can be blinded in randomized controlled clinical trials assessing the efficacy of acupuncture. Ideal sham acupuncture should not be distinguishable from an actual acupuncture treatment provided to the experimental group to ensure patient blinding; additionally, it should not have any physiological or biological effect. Providing evidence for such sophisticated sham acupuncture devices is critical, as control settings in clinical studies are based on research verifying their validity.
Methods
Three core electronic databases - PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials - will be used to search for validity verification studies of sham acupuncture devices. Clinical studies that verify the validity of non-penetrating sham acupuncture devices will be included in the review.
Results
The study design, participant information, experimental and control groups, study population’s experience with acupuncture, outcome variables, and results of studies that verify the validity of sham acupuncture devices will be systematically reviewed.
Conclusion
This systematic review of validity verification studies of sham acupuncture devices is expected to help the development of more sophisticated sham acupuncture, as well as the design of studies verifying its validity in the future.
Review Articles
- Minimum Clinically Important Difference for Nonsurgical Interventions for Spinal Diseases: Choosing the Appropriate Values for an Integrative Medical Approach
- Ye-Seul Lee, Sungmin Lee, Yoon Jae Lee, In-Hyuk Ha
- Perspect Integr Med. 2023;2(2):86-99. Published online June 23, 2023
- DOI: https://doi.org/10.56986/pim.2023.06.003
- 717 View
- 26 Download
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material 
- The minimal clinically important difference (MCID) plays a crucial role in the design and interpretation of clinical trials, as it helps in distinguishing between statistically significant and clinically meaningful outcomes. This scoping review aims to collate and appraise the current research concerning the validation of MCIDs for surgical and nonsurgical measures for spine disorders. Two databases of MEDLINE (PubMed and EMBASE) were searched. There were 1,590 studies retrieved and 79 were selected as eligible for review. Measurement tools such as the Oswestry Disability Index, Neck Disability Index, Numeric Rating Scale, and Visual Analogue Scale were assessed by regions and interventions. A total of 24 studies identified MCIDs on nonsurgical interventions, and 55 studies identified MCIDs on surgical interventions. The range of MCIDs varied greatly depending on study population, specific interventions, calculation methods, and outcomes. This scoping review emphasizes the complexity and variability in determining MCIDs for musculoskeletal or neurodegenerative spinal diseases, influenced by several factors including the intervention type, measurement tool, patient characteristics, and disease severity. Given the wide range of reported MCIDs, it is crucial to consider the specific context when interpreting these values in clinical and research settings. To select an appropriate MCID value for comparison in a clinical trial, careful consideration of the patient group, intervention, assessment tools, and primary outcomes is necessary to ensure that the chosen MCID aligns with the research question at hand.
- A Review of Major Secondary Data Resources Used for Research in Traditional Korean Medicine
- Chunhoo Cheon, Bo-Hyoung Jang, Seong-Gyu Ko
- Perspect Integr Med. 2023;2(2):77-85. Published online June 23, 2023
- DOI: https://doi.org/10.56986/pim.2023.06.002
- 1,110 View
- 23 Download
- 2 Citations
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 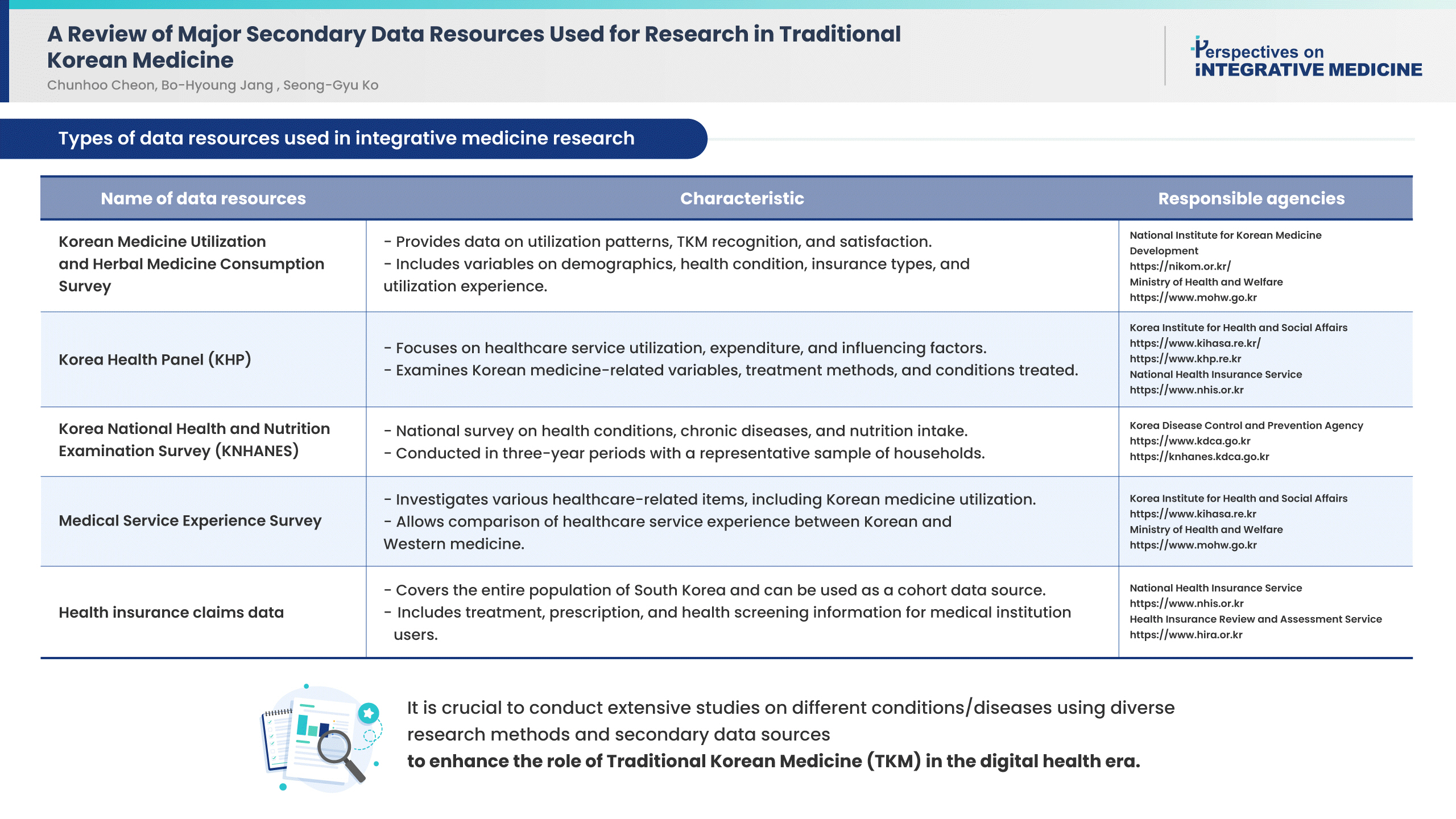
- Research in health care using secondary data is steadily increasing worldwide. In this study, secondary healthcare data was reviewed, so that the information can potentially be used for Korean medicine research. The characteristics of the data, including the variables related to Korean medicine and the method of obtaining data, were summarized. The Korean medicine variables were extracted from the Korean Medicine Utilization Survey, Korea Health Panel, Korea National Health and Nutrition Examination Survey, Medical Service Experience Survey, and the health insurance claims data. Except for health insurance claims data, the data was obtained through relatively simple procedures. There were differences in the characteristics of each secondary data and the extent to which it was used in Korean medicine research. Many Korean medicine studies using secondary data will be conducted in the future and researchers must understand the characteristics of the data and analyze it appropriately.
-
Citations
Citations to this article as recorded by- Real-world data analysis on effectiveness of integrative therapies: A practical guide to study design and data analysis using healthcare databases
Ye-Seul Lee, Yoon Jae Lee, In-Hyuk Ha
Integrative Medicine Research.2023; 12(4): 101000. CrossRef - Trends in the treatment of fibromyalgia in South Korea between 2011 and 2018: a retrospective analysis of cross-sectional health insurance data
Jin-Sil Yu, Eun-San Kim, Kyoung Sun Park, Yoon Jae Lee, Yeon Cheol Park, Dongwoo Nam, Eun-Jung Kim, In-Hyuk Ha
BMJ Open.2023; 13(12): e071735. CrossRef
- Real-world data analysis on effectiveness of integrative therapies: A practical guide to study design and data analysis using healthcare databases
- A Scoping Review of Clinical Research on Motion Style Acupuncture Treatment
- Doori Kim, Yoon Jae Lee, In-Hyuk Ha
- Perspect Integr Med. 2023;2(2):65-76. Published online June 23, 2023
- DOI: https://doi.org/10.56986/pim.2023.06.001
- 1,691 View
- 41 Download
- 2 Citations
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 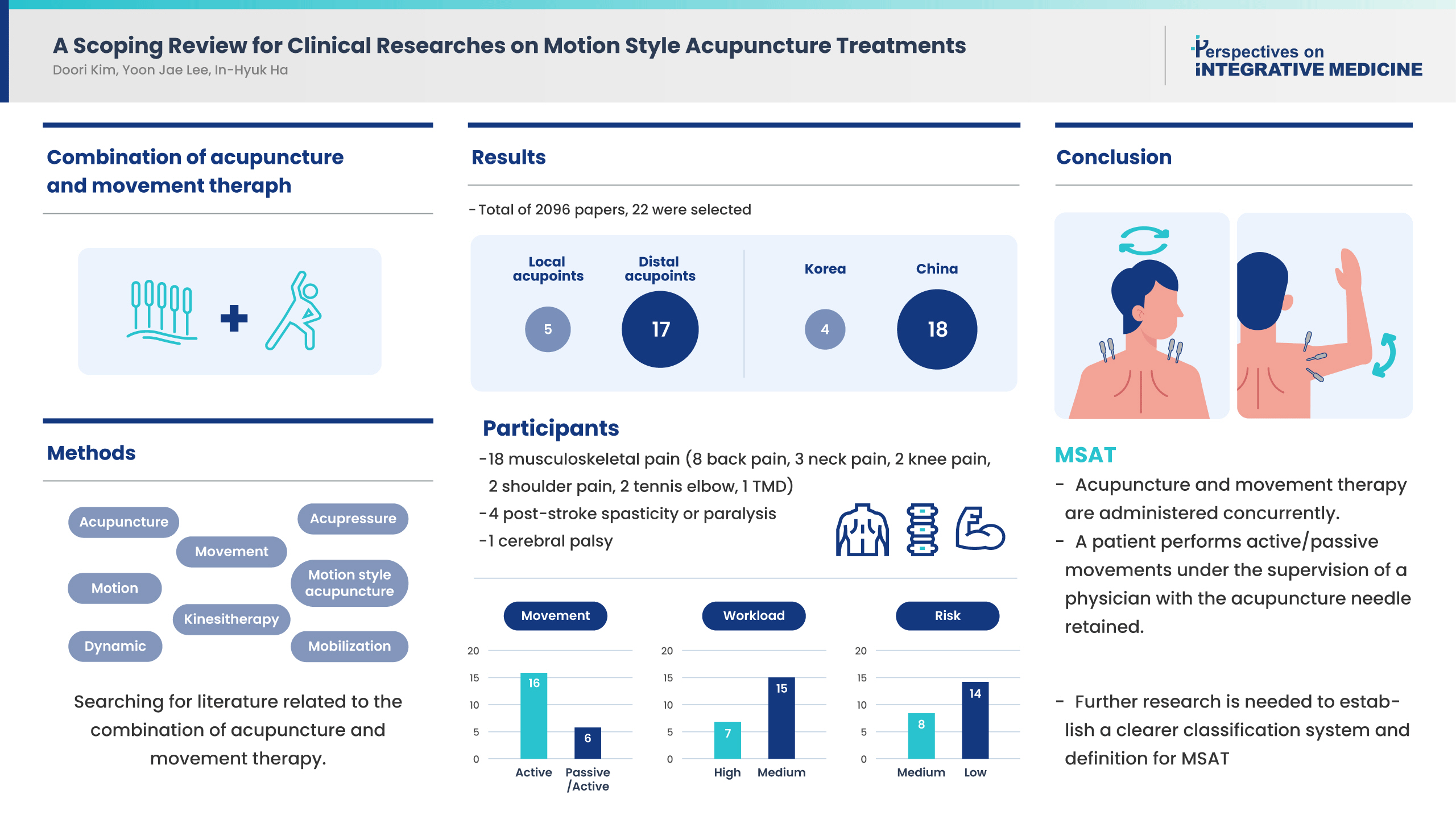
- This scoping review was conducted to examine the concept of Motion style acupuncture treatment (MSAT), use in clinical practice, its effectiveness, and safety. A literature review of clinical study treatment methods combining acupuncture and movement therapy was performed using PubMed. Of 2,096 studies retrieved, 22 were included in this review. There were 12 randomized controlled trials, and all 22 studies were published in China and Korea, mostly, within the last 3 years. There were five studies concerning local acupoints and 17 studies regarding needling at distal acupoints, and the level of risk of the procedure was “high” in eight studies and “moderate” in 14 studies. The study participants were patients with musculoskeletal pain, and many studies reported significant improvements in pain and functional disability outcomes following treatment using MSAT. For conclusion, MSAT refers to a treatment method in which a patient performs active/passive movements under the supervision of a physician with the acupuncture needle retained at the insertion site. However, there are a limited number of MSAT studies, and various treatment types and related terms are mixed. Further studies, classification of the types of MSAT using a well-established classification system, and a clearer definition of the MSAT concept are needed.
-
Citations
Citations to this article as recorded by- Effectiveness of lumbar motion style acupuncture treatment on inpatients with acute low back pain: A pragmatic, randomized controlled trial
Oh-Bin Kwon, Dong Wook Hwang, Dong-Hyeob Kang, Sang-Joon Yoo, Do-Hoon Lee, Minjin Kwon, Seon-Woo Jang, Hyun-Woo Cho, Sang Don Kim, Kyong Sun Park, Eun-San Kim, Yoon Jae Lee, Doori Kim, In-Hyuk Ha
Complementary Therapies in Medicine.2024; 82: 103035. CrossRef - Effectiveness and Safety of Progressive Loading–Motion Style Acupuncture Treatment for Acute Low Back Pain after Traffic Accidents: A Randomized Controlled Trial
Seung-Yoon Hwangbo, Young-Jun Kim, Dong Guk Shin, Sang-Joon An, Hyunjin Choi, Yeonsun Lee, Yoon Jae Lee, Ju Yeon Kim, In-Hyuk Ha
Healthcare.2023; 11(22): 2939. CrossRef
- Effectiveness of lumbar motion style acupuncture treatment on inpatients with acute low back pain: A pragmatic, randomized controlled trial
Protocol
- A Protocol for the Overview of Systematic Reviews of Aromatherapy for Management of Health
- Ki Jung Kil, Myeong Soo Lee
- Perspect Integr Med. 2023;2(1):56-58. Published online February 21, 2023
- DOI: https://doi.org/10.56986/pim.2023.02.008
- 900 View
- 32 Download
-
 Abstract
Abstract
 PDF
PDF - Background
Aromatherapy has been reported to have a positive effect on various health conditions. While these studies show positive results, many of them have limited evidence. The aim of this study was to develop a protocol to evaluate all systematic reviews (SRs) that have evaluated the efficacy of aromatherapy (for any health condition) as a therapeutic treatment (protocol registration number INPLASY202280089).
Methods
We will include aromatherapy through different therapeutic application methods such as inhalation, massage, and bathing. Seven international databases (including PubMed, AMED, EMBASE, the Cochrane Library), and three Korean medical databases (Korean Studies Information, Research Information Service System, KoreaMed), will be searched. The SR process, including study selection, data extraction, and assessment, will be performed by two independent reviewers. Methodological assessment will be performed using AMSTAR-2.
Discussion
The benefits of aromatherapy for health management are evaluated to provide useful information to patients and therapists and inform decisions on further studies on this topic.
Review Articles
- Trends in the Development of Sham Acupuncture-Related Technologies Focused on Patents Applied for in South Korea
- Sung Min Lim
- Perspect Integr Med. 2023;2(1):36-41. Published online February 21, 2023
- DOI: https://doi.org/10.56986/pim.2023.02.005
- 1,730 View
- 21 Download
- 2 Citations
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 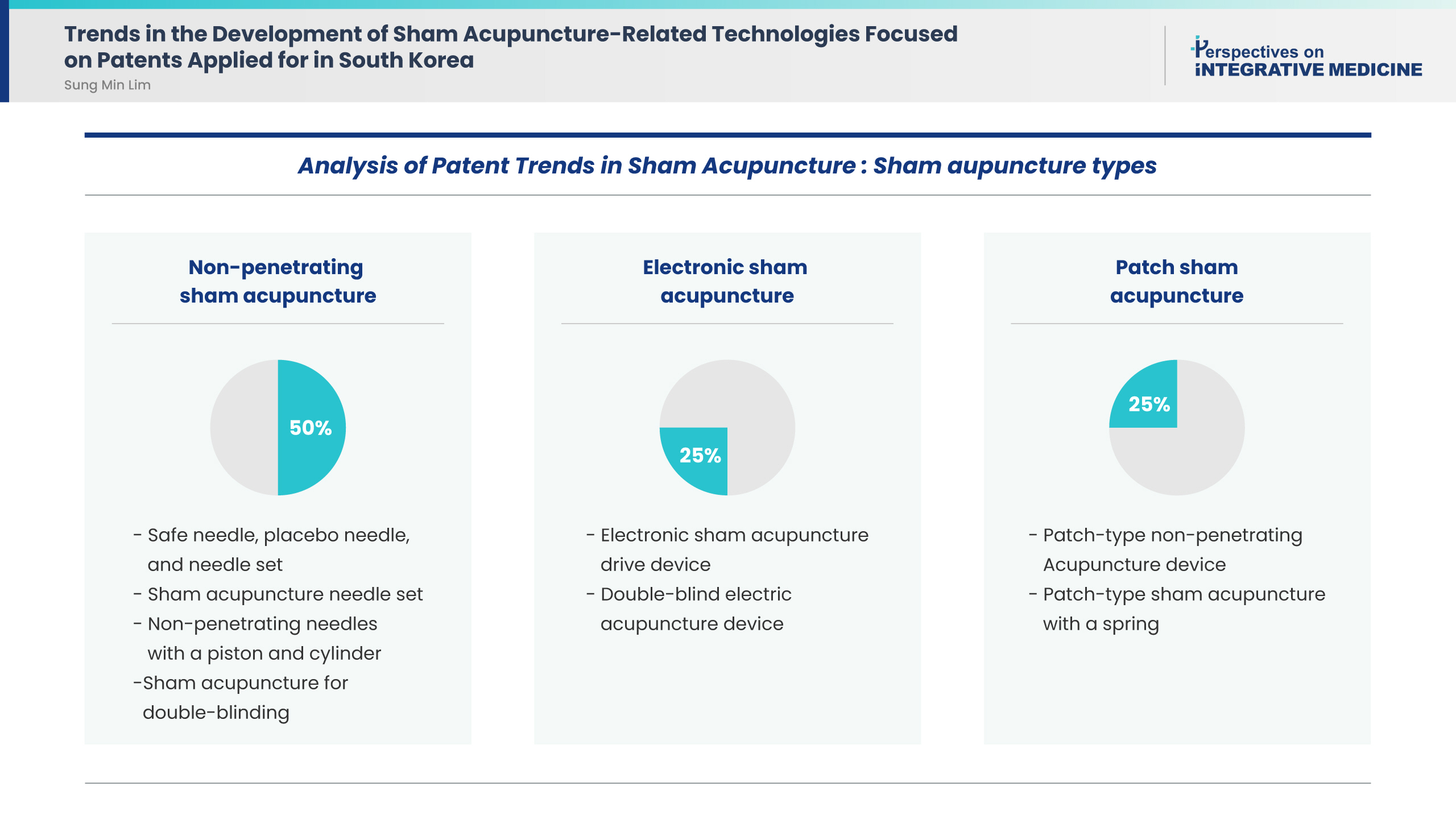
- Background
This study aimed to analyze the trends in Korean patents for sham acupuncture.
Methods
The electronic database of the Korea Intellectual Property Rights Information Service was searched for Korean patents for sham acupuncture from inception till September 2020. Patents, which were not related to sham acupuncture, were excluded. The applicant, application date, International Patent Classification, and technological content of sham acupuncture were analyzed.
Results
This study included eight patents. Application analysis identified the following sham acupuncture types: four (50%), two (25%), and two (25%) patents were for non-penetrating sham acupuncture, electronic sham acupuncture, and patch sham acupuncture, respectively. All patents aimed to use sham acupuncture as a control for rigorous double-blind clinical trials to verify the efficacy of real acupuncture treatment.
Conclusion
The present findings suggest that technological advances were focused on developing various types of sham acupuncture methods for double-blind studies. Further large-scale studies using rigorous designs are needed to investigate new sham acupuncture applications. -
Citations
Citations to this article as recorded by
- Proposal of a Case Reporting Draft Guideline for Pharmacopuncture: Literature Review of Pharmacopuncture Case Reports
- Soohyun Jeong, Chaeyeon Son, Hyunjin Kim, Gapsik Yang, Hongmin Chu, Jungtae Leem
- Perspect Integr Med. 2023;2(1):24-35. Published online February 21, 2023
- DOI: https://doi.org/10.56986/pim.2023.02.004
- 1,354 View
- 38 Download
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material 
- Pharmacopuncture is a popular treatment that combines the advantages of both herbal medicine and acupuncture. However, pharmacopuncture care reporting guidelines have not yet been developed. This study aimed to propose a reporting guideline draft for pharmacopuncture case reports. Pharmacopuncture case reports were retrieved from 4 databases (KCI, RISS, ScienceON, OASIS) to analyze the items reported and their fidelity. We analyzed 5 existing reporting guidelines related to Korean medicine case reporting to identify the items to be included in the extension of pharmacopuncture reporting guidelines. From 3,684 studies, 29 case reports were included and 4 items were identified as not reported in enough detail: “direction and depth of pharmacopuncture” (89.5%); “method of manufacturing the syringe needle” (82.8%); “posture of the patient during the therapy” (75.9%); and “pharmacopuncture recipe” (69.5%). As a result of analyzing moxibustion and acupuncture clinical trial reporting guidelines, it was determined that detailed reporting guidelines on the type of pharmacopuncture, manufacturing method, and treatment method were required and we propose that a pharmacopuncture reporting guideline draft should include these details. Further investigations are warranted using the Delphi technique to reach agreement with clinical practitioners and clinical research experts.



 First
First Prev
Prev


